FDA Approval
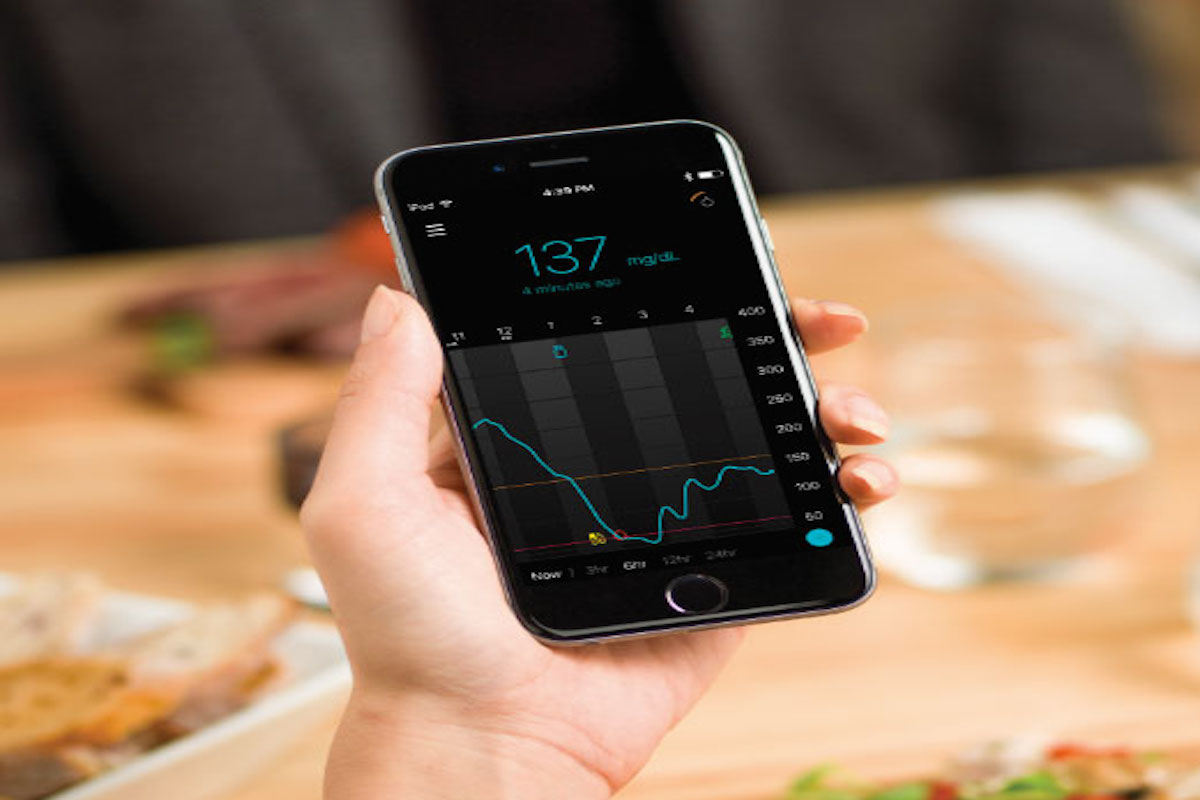
Medtronic recently announced FDA approval of its Guardian Connect mobile Continuous Glucose Monitor (CGM), along with the paired Guardian Connect App for Apple iOS devices. A limited US launch is expected between May-July 2018, with wider availability in August-October.
Guardian Connect is Medtronic’s “standalone,” real-time, mobile CGM in the US, meaning it does not send data to a paired pump – real-time CGM information goes straight to a smartphone app, similar to Dexcom’s G5.
 Enjoy 15% OFF members only
Enjoy 15% OFF members only